Our paper entitled “A force-induced directional switch of a molecular motor enables parallel microtubule bundle formation” has been published in Cell.
Microtubule networks are essential component of the cytoskeleton required for the cell growth and cell division. Generally, microtubules originate from microtubule organization centers (MTOC), such as centrosomes. The nucleation itself does not provide any specific direction to the microtubule growth; rather microtubules grow autonomously in all directions and subsequent molecular events dynamically rearrange microtubules growing from MTOC into parallel bundles. However, how cells control the direction of the growth of individual microtubules from a MTOC remained unknown.
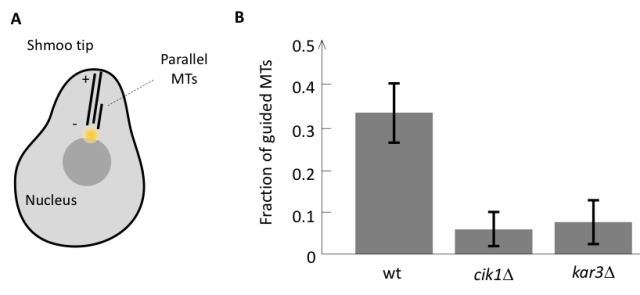
We approached this question by first identifying the components responsible for the parallel microtubule growth in a model system. During mating budding yeast cells grow a mating protrusion, which is based on a parallel microtubule bundle consisting of only a handful of microtubules. We have found that as soon as there is one microtubule pointing to the shmoo tip it is likely that the growth of another microtubule would be guided along the existing one leading to the formation of a parallel bundle (Figure 1A). However, in mutant cells lacking minus end directed motor Cik1-Kar3 from kinesin-14 family, all microtubules were growing independently and parallel growth did not occur, suggesting that Cik1-Kar3 is required for parallel bundle formation in yeast (Figure 1B).
Our further analysis revealed that this activity of kinesin-14 is likely to depend on its interaction with microtubule plus-end tracker EB1, and in order to investigate whether two components Cik1-Kar3 and EB1 are sufficient for parallel microtubule growth we have set up an in vitro assay with artificial centers that nucleate microtubule in all direction similar to in vivo.
Our data showed that in the presence of EB1, Cik1-Kar3 localizes to microtubule ends. Microtubule polymerization switches the direction of movement of Cik1-Kar3 bound to microtubule tips and promotes bundles formation by parallel microtubule growth (Figure 2A,B). Interestingly, we found that microtubule growth decreases with increasing amount of Cik1-Kar3 at growing microtubules (Figure 2C) suggesting that minus-end directed movement of Cik1-Kar3 might be playing tug-of-war with the growing microtubule end.

In order to investigate molecular mechanism underlying ability of Cik1-Kar3 to change the direction of movement we used optical trap to track the movement of single kinesin molecules with high temporal and spatial resolution. Our recordings showed that the motor moved to the minus end with discrete ~8 nm steps and frequently made backward steps (Figure 3A). In the presence of external forces as small as a fraction of a pico-newton, backward steps were favored effectively reversing the direction of the motor (Figure 3B), suggesting that polymerizing microtubule can switch the direction of Cik1-Kar3 movement.

Finally, we asked whether human and Drosophila EB1/kinesin-14 system could support parallel microtubule growth similar to yeast proteins. We have purified fluorescently labeled components and showed that although efficiency of human and Drosophila proteins were lower, they did support bundle formation by parallel microtubule growth (Figure 4).

Our study showed a mechanism for the effective generation of parallel microtubule bundles from a MTOC by the collective action of just two conserved molecular components EB1 and kinesin-14 in diverse organisms (Figure 5). We found that this mechanism is based on the unusual activity of kinesin-14 molecules that allow them to move processively and change the direction of movement due to the force applied by a polymerizing microtubule. This mechanism relies on diffusive interactions between kinesin-14 and microtubule lattice and does not depend on the widely established mechanism based on the kinesin-14 stalk rotation.

The proposed mechanism may have very general relevance for a broad range of functions during cell division, growth and migration. The essence of the mechanism lies in the ability of the kinesin-14 to switch the direction under opposing force, and it might represent a general feature that allows distinct functionalities not available by other molecular motors.
Relevant publication:
Maxim I. Molodtsov, Christine Mieck, Jeroen Dobbelaere, Alexander Dammermann, Stefan Westermann, and Alipasha Vaziri,
A force-induced directional switch of a molecular motor enables parallel microtubule bundle formation
Cell 176, 539 (2016).
(Download)
