Our paper entitled “Volumetric Ca2+ Imaging in the Mouse Brain using Hybrid Multiplexed Sculpted Light (HyMS) Microscopy” has been published in Cell.
We have developed a modular platform named Hybrid Multiplexed Sculpted Light (HyMS) microscopy featuring a systems-wide design paradigm that maximizes the acquisition volume size and speed while maintaining fidelity for obtaining single neuron activity traces. Our modular design utilizes a hybrid two- and three-photon acquisition and allows for volumetric recording of neuroactivity at single-cell resolution within up to 1 × 1 × 1.22 mm volumes at up to 17 Hz in awake behaving mice. We establish the capabilities and potential of the different configurations of HyMS microscopy at depth and across brain regions by applying it to in-vivo recording of up to 12,000 neurons in mouse auditory cortex, posterior parietal cortex and hippocampus.
We demonstrate the performance of HyMS microscopy and its different configurations (Fig. 1) in the mouse posterior parietal cortex, primary auditory cortex, and the hippocampus.
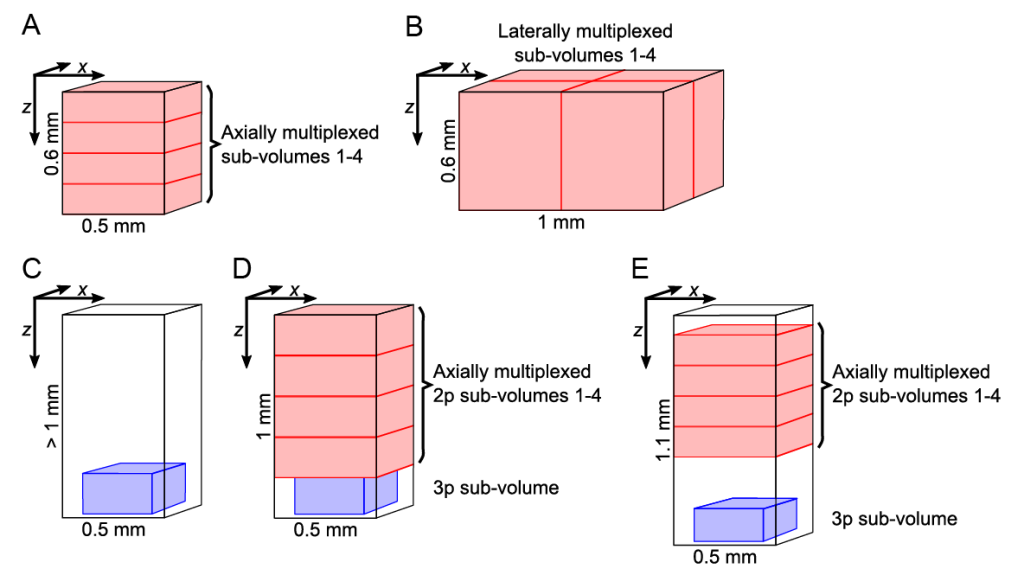
Figure 1| Different acquisition configuration of the HyMS microscopy
Our target volumes span axially over cortical layers 1-6, encompassing basal dendritic and pyramidal layers of the hippocampus CA1 region, within the hippocampus from CA1 to the dentate gyrus, and cover laterally up to 1 mm2. In our different configurations, we show calcium (Ca2+) of neurons within volumes of up to 1 × 1 × 0.6 mm at >5 Hz, or 340 × 340 × 250 µm located at ~1 mm depth, or ~0.7 × 0.7 × 1 mm spanning an entire cortical column and a major portion of the hippocampus. Further we have pushed the attainable functional high-speed imaging depth to >1.2 mm. Our method has enabled near-simultaneous in vivo recording from up to 12,000 neurons across different regions and depths of the mouse brain. Thereby it opens up new possibilities to study how distributed network dynamics of large neuron populations encode sensory inputs and generate behavior.
Video 1 | Example 4x axial volumetric 2p-MuST Ca2+ imaging in mouse PPC. 3D rendering (MIP) of a 30-min recording in mouse PPC, 690 × 675 × 600 µm volumetric field of view at 16.7 Hz, cytosolic GCaMP6f. The video shows the first 3 min of the recording. Playback speed: 3×.
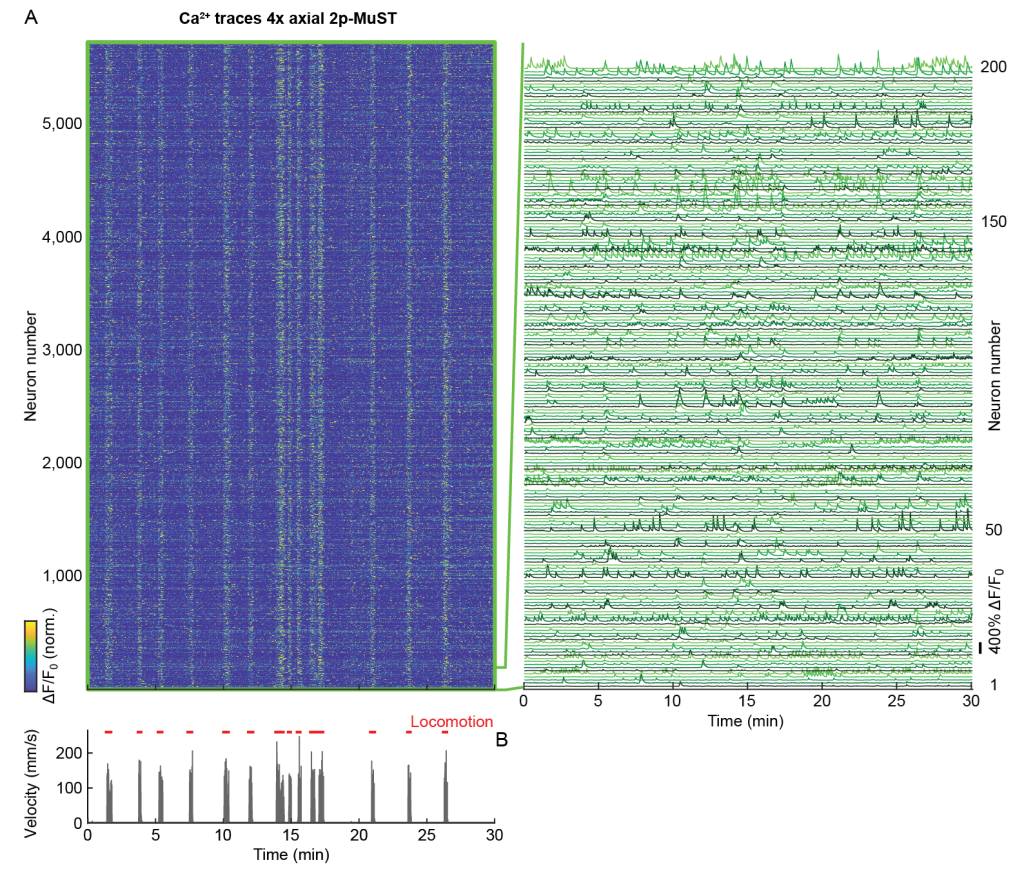
Figure 2 | High-speed volumetric 4x-axial 2p-MuST imaging of mouse PPC
In the 4x axial (Multiplexed Scanned Temporal Focusing) MuST configuration, we recorded over a 600 µm axial range with each of the four beamlets covering a 150 µm sub-volume (Video 1). Using this configuration, we recorded within a volume of ~690 × 675 × 600 µm at 16.7 Hz (Fig. 2A). Depending on the recording duration (~10-30 min) and the animal’s behavioral state, we typically found ~3,000-6,000 active neurons in our imaging volume (Fig. 2).
Video 2 | Example of 4x lateral 2p-MuST recording of mouse primary auditory cortex. 3D rendering of a 20-min volumetric 4x-lateral 2p-MuST recording in mouse primary auditory cortex, 1,000 × 1,000 × 600 µm FOV, 5.1 Hz, double-transgenic GCaMP6s. The video shows the first 3 min of the recording. Playback speed: 3×.
To demonstrate the broader application of 2p-MuST, we configured it for 4x lateral multiplexing (Fig. 1B) and applied it to the mouse auditory cortex. We recorded a 1 × 1 × 0.6 mm volume at 5.1 Hz and identified 11,907 active neurons in a 20 min recording (Video 2) where we presented the animal with frequency sweeps across its hearing range. The application of 2p-MuST to the primary auditory cortex demonstrates how the ability to capture the dynamics of thousands of neurons within a volume of 0.6 mm3 can be used to study the functional organization, and in particular the columnar structure, of large circuits.
Using our optimized 3p imaging configuration, allowed us to perform high-speed single-plane and volumetric 3p recording at depths up to 1.22 mm below the brain surface (Video 3).
Video 3 | Video 3: Example single-plane 3p recording of mouse hippocampus CA1 through intact cortex. Single-plane 3p recording in mouse hippocampus CA1 through intact cortex at 1,160 µm depth, 340 × 340 µm FOV, 78 Hz, cytosolic GCaMP6s. Left: Unprocessed raw dataset (except for motion correction using NoRMCorre). Right: Denoised dataset. Scalebar: 100 µm. The video shows the first 5 min of the recording. Playback speed: 5×.
We could also demonstrate, to our knowledge for the first time, volumetric Ca2+ imaging at 750-1,000 µm tissue depths of a 340 × 340 × 250 µm volume at 3.9 Hz (Video 4).
Video 4 | Example volumetric 3p recording of mouse PPC layer 6. 3D rendering of a 5-min volumetric 3p recording in mouse PPC L6 through intact cortex at 750-1,000 µm depth, 340 × 340 × 250 µm FOV, 3.9 Hz, cytosolic GCaMP6f. Playback speed: 5×.
HyMS microscopy is capable of accessing brain regions residing below the neocortex such as the hippocampus, overcoming the limitations forced by cortical aspiration of the overlying structures that may have critical inputs or fibers of passage to the target volume. However, the versatility of HyMS microscopy also allowed us to place the 3p acquisition volume even deeper below the 2p-MuST volumes and thereby record simultaneously from PPC and the underlying hippocampus CA1. To demonstrate this capability, we chose the sub-volumes such that 2p-MuST accessed neurons were located within 100-700 µm axial range while we simultaneously recorded from neurons that were located within 950-1,100 µm depth range using volumetric 3p imaging (Fig. 3 and Fig. 1E). Thereby, we could record from ~3,500 active neurons within the PPC column and hippocampus CA1 neurons located directly underneath at 4.6-13.8 Hz (Fig. 3).
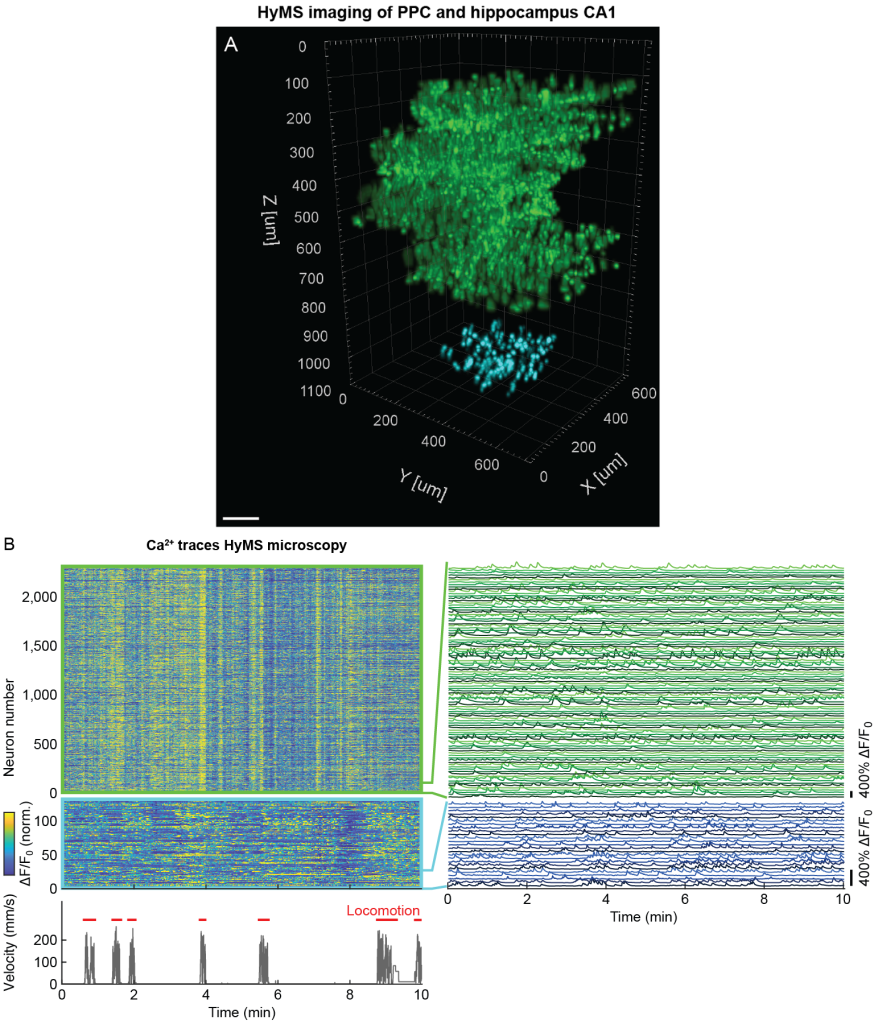
Figure 3 | Simultaneous volumetric imaging of mouse PPC layers 1-5 and hippocampus CA1 using HyMS
The capabilities of HyMS microscopy together with the ongoing progress in optogenetics provide new opportunities to gain insights into the computational principles of information processing in the mammalian brain and amongst others to experimentally test a range of proposed theoretical models for computations.
Relevant publication:
Siegfried Weisenburger, Frank Tejera, Jeffrey Demas, Brandon Chen, Jason Manley, Fraser T. Sparks, Francisca Martínez Traub, Tanya Daigle, Hongkui Zeng, Attila Losonczy, and Alipasha Vaziri
Volumetric Ca2+ Imaging in the Mouse Brain using Hybrid Multiplexed Sculpted Light (HyMS) Microscopy
Cell 177, 1-17 (2019).
(Download)
