We have developed strategies for optimizing of the present tradeoffs in light-sculpting microscopy by evaluating various scanning modalities theoretically and experimentally and identifying approaches to maximize the obtainable volume speeds, and depth penetration in scattering brain tissue using different laser systems.
To understand how dynamics of neuronal networks are related to brain function tools that allow capturing the functional dynamics of large cellular populations at high speed are required. One approach is based on temporal focusing (TeFo). We have recently applied wide-field TeFo as a technique for high-speed brain-wide calcium imaging in C. elegans. (Links: Paper or Overview) The effective decoupling of axial and lateral confinement of the excitation beam in TeFo allows to excite laterally wide yet axial confined volumes which in turn allow the use of parallel, camera-based detection schemes. While using this approach a large area can be in principle excited simultaneously the actual increase in effective frame (or volume) rate also depends on the available laser power, pulse repetition rate and peak pulse energy. Moreover, such a wide-field approach to volumetric imaging may also poses technical limitations due to the required high peak power, which can usually only be provided by amplified laser systems. Finally the parallel detection scheme via cameras poses limitations on the obtainable depth due to scattering which leads to cross talk between neighboring pixels.
In this work we have addressed some of these shortcomings. First, we investigate the present tradeoffs in light-sculpting microscopy both theoretically and experimentally, evaluating various scanning modalities and devising strategies to maximize the obtainable volume speeds, and depth penetration in brain tissue based on the experimental requirements. Doing so we found that the imaging field-of-view can be increased to >200×200 µm2, while retaining a physiologically relevant imaging speed of 10 ms/plane by using line- or spiral-scanning methods in combination with temporal focusing (see Fig.1).
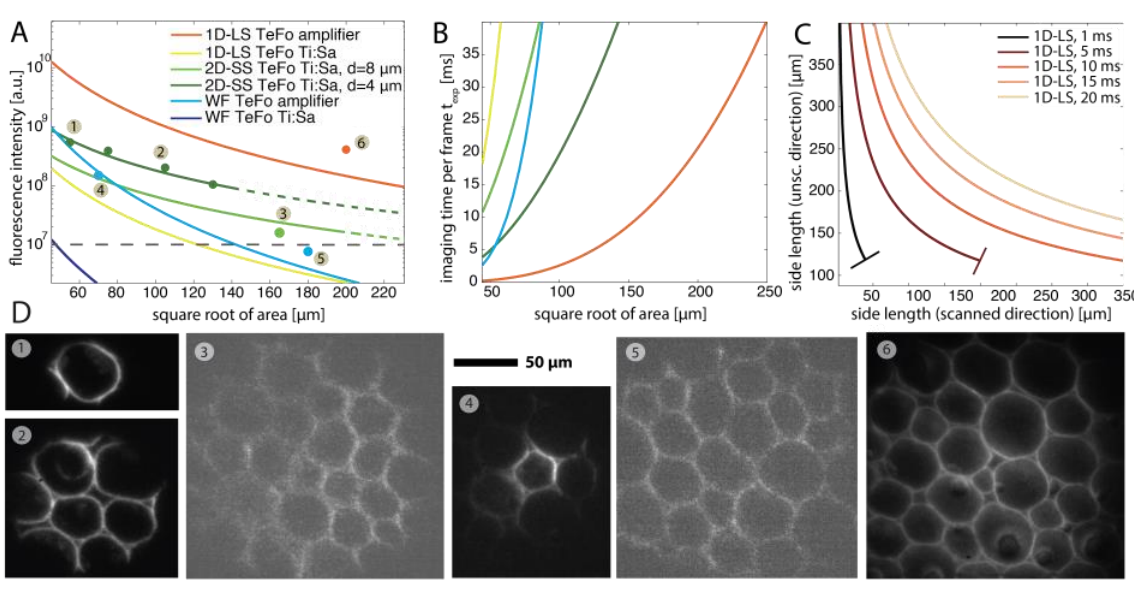
In particular, we also found that one can achieve an imaging FOV of ~150 µm diameter at this imaging speed using galvanometer mirrors and a standard pulsed Ti:Sa laser instead of an amplifier laser system. The experimental setup, the various scanning modalities is shown in Fig. 2 and typical experimental results can be seen in Fig. 3.
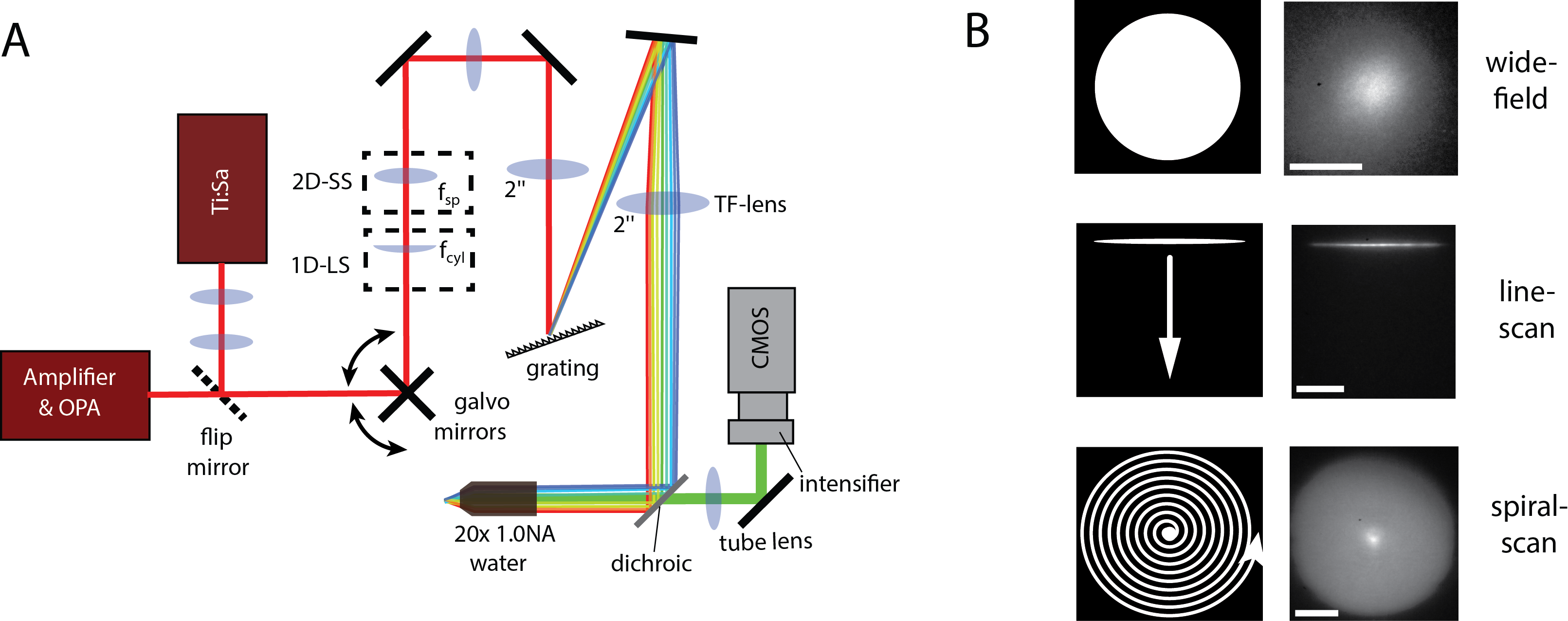
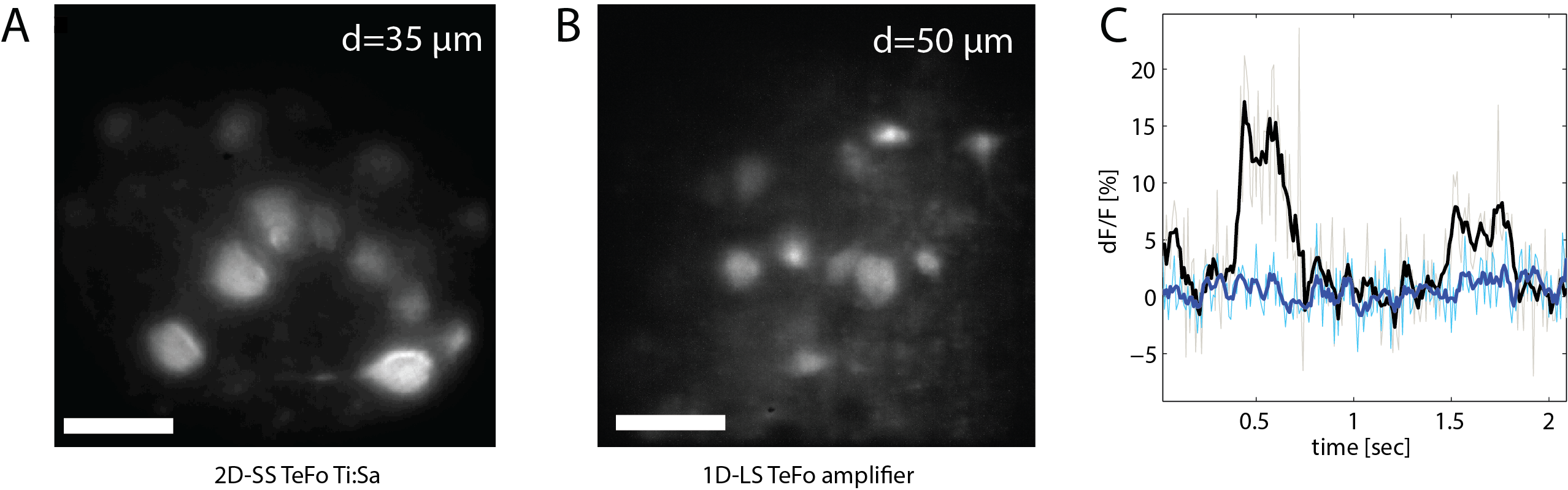
Furthermore, in order to suppress the detrimental effects of scattering we used the line-scan TeFo modality in combination with a synchronized rolling shutter read-out mode of our sCMOS camera, in which the detection area of the camera is restricted to a small line that moves synchronously with the illuminated line in the sample. The reduction of the active sensor area in one dimension to the width of a line allows to detect photons originating from the line-shaped illumination area on the sample only, while rejecting scattered photons. Again, we theoretically evaluated the expected effects of scattering reduction at different depths using Monte Carlo simulations, and then validated our theoretical predictions experimentally in acute mouse brain slices expressing GCaMP6m.
Relevant publication:
P. Rupprecht, R. Prevedel, F. Groessl, W. Haubensak, & A. Vaziri
Optimizing and extending light-sculpting microscopy for fast functional imaging in neuroscience
Biomedical Optics Express 6:2, 353-368 (2015). (Download)
