Our paper entitled “High-speed volumetric imaging of neuronal activity in freely moving rodents” has been published in Nature Methods.
We present a head-mounted miniaturized light-field microscope (MiniLFM) capable of capturing neuronal network activity within a volume of 700 × 600 × 360 µm at 16 Hz frame rate in the hippocampus of freely moving mice. We demonstrate that neurons as close as ~15 µm to each other and at depths up to 360 µm can be discriminated. MiniLFM is based on Light Field Microscopy (LFM), a 3D imaging technique that our group has established in a previous publication as a versatile neural recording technique, and the Seeded Iterative Demixing (SID) algorithm, which we presented recently, and which extends LFM into more strongly scattering tissue such as the mammalian cortex.
To understand the neuronal basis of complex and ethologically relevant behavior, fast, depth-penetrating volumetric imaging techniques are required that are compatible with free behavior and social interaction. Currently, volumetric Calcium (Ca2+) imaging techniques capable of extracting information from the mammalian or avian brain require head fixation.
We overcame the aforementioned limitations by combining the existing head-mounted miniature microscope (“Miniscope“) platform with Light Field Microscopy-based (LFM) detection and a computational strategy based on constrained matrix factorization (Seeded Iterative Demixing, “SID”). LFM allows capturing volumetric information in a single exposure of a 2D image sensor, while SID extends the reach of LFM into the scattering mammalian brain.
The head-mounted MiniLFM module incorporates only one additional optical element compared to the Miniscope platform, and is portable by an adult mouse, allowing it to move freely in an arena (Fig. 1).
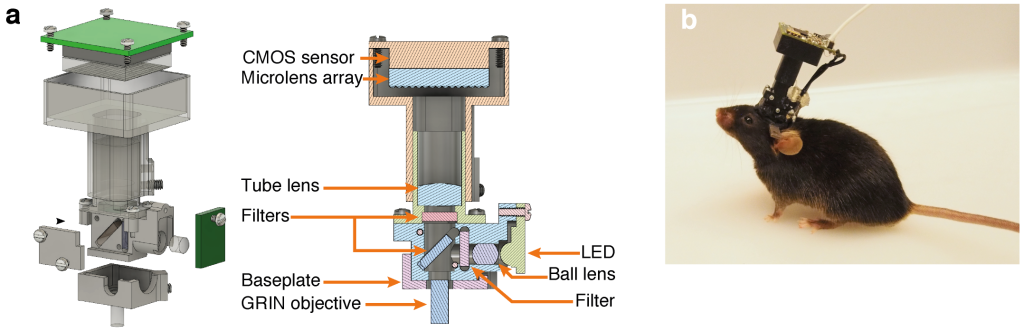
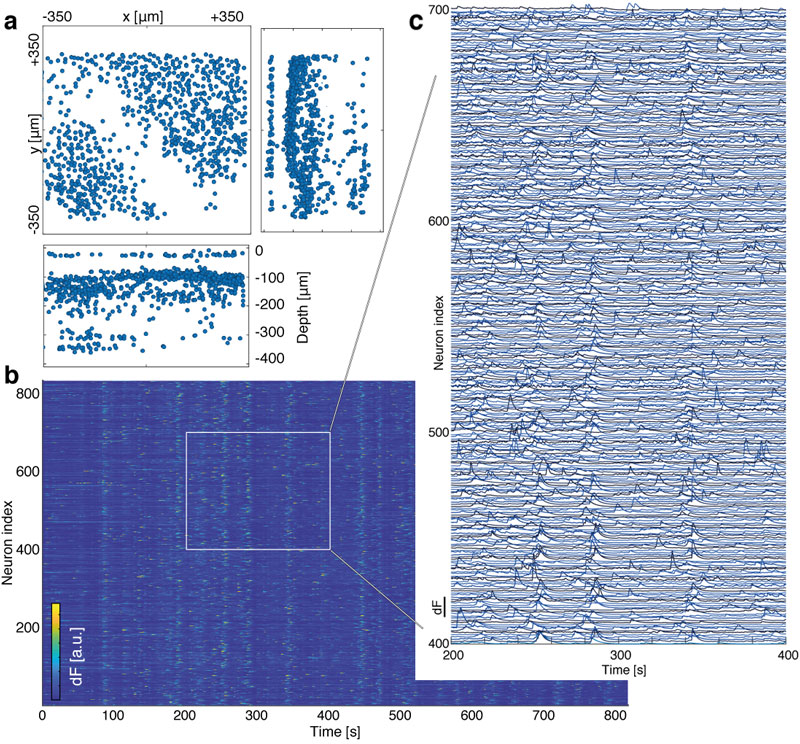
We verified the performance of our MiniLFM by recording spontaneous volumetric activity of hippocampal CA1 neurons in freely moving mice. While the raw MiniLFM frames appear blurred and do not allow the identification of individual neurons, the SID algorithm extracts neuronal positions and corresponding activity time series in the CA1 pyramidal and Stratum radiatum layers down to a depth of 360 µm (Fig. 2). Moreover, volumetric recording with our method reveals the shape of the pyramidal layer clearly through a 3D rendering of our recording volume (see video below).
Video 1 | Perspective rendering of neuron positions and Ca2+ activity during free movement, recorded using MiniLFM from mouse hippocampus CA1 and extracted using SID algorithm. Recording frame rate: 16 Hz. Real-time recording duration: 30 min. Playback speed: 10.7×. White dashed lines indicate field-of-view of MiniLFM with dimensions 800 × 800 × 400 µm. Neurons are rendered as spheres placed at their SID-extracted locations. Color indicates neuron brightness.
We identified temporal signals corresponding to the 807 active neurons in our 26-minute example recording (Fig. 2). We found the typical shapes of Ca2+ transients as observed by other methods to be reproduced faithfully, even for neurons at depths of ~360 µm. We were able to demonstrate good agreement between MiniLFM / SID data and the simultaneously recorded ground truth data (using a tabletop two-photon microscope).
Overall, our MiniLFM design – which combines LFM, SID and Miniscope technology – enables fast volumetric imaging at low photobleaching and phototoxicity in scattering tissue of freely moving animals. Our MiniLFM design establishes a simple and extensible platform that can be customized and adapted to other animals. Together with the computational efficiency and neuron discrimination capability of the SID algorithm, our approach offers a platform for population-level studies of neural information processing in freely behaving animals and opens the door to the analysis of the neuronal basis of social interaction.
Relevant publication:
Oliver Skocek, Tobias Nöbauer, Lukas Weilguny, Francisca Martínez Traub, Chuying Naomi Xia, Maxim I. Molodtsov, Abhinav Grama, Masahito Yamagata, Daniel Aharoni, David D. Cox, Peyman Golshani, and Alipasha Vaziri
High-speed volumetric imaging of neuronal activity in freely moving rodents
Nature Methods 15, 429–432 (2018).
(Download)
