Year: 2015
December 21, 2015
We congratulate her on the PhD title awarded by the university of Singapore. Previously, she worked on neurobehavioral manifestations of decision…November 13, 2015
We welcome Jeroen Delcour (Netherlands) and Ren Zicheng (China) as visiting scientists in our group. They will try to understand…May 18, 2015
Together with our colleagues at the Institute for Biophysical Dynamics at the University of Chicago, we have developed a method…May 18, 2015
Together with our collaborator Markus Arndt we published in Analytical Chemistry on how to improve Laser-induced acoustic desorption (LIAD) for…March 23, 2015
We congratulate Dip. Ing. Magdalena Helmreich for an excellent defense of her thesis and graduation with distinction!March 9, 2015
Michael got his PhD in Physics from the University of Queensland, Australia, where he worked on new experimental strategies for optical tweezers.…February 3, 2015
Motors proteins of the conserved kinesin-14 family have important roles in mitotic spindle organization and chromosome segregation. Previous studies have…January 12, 2015
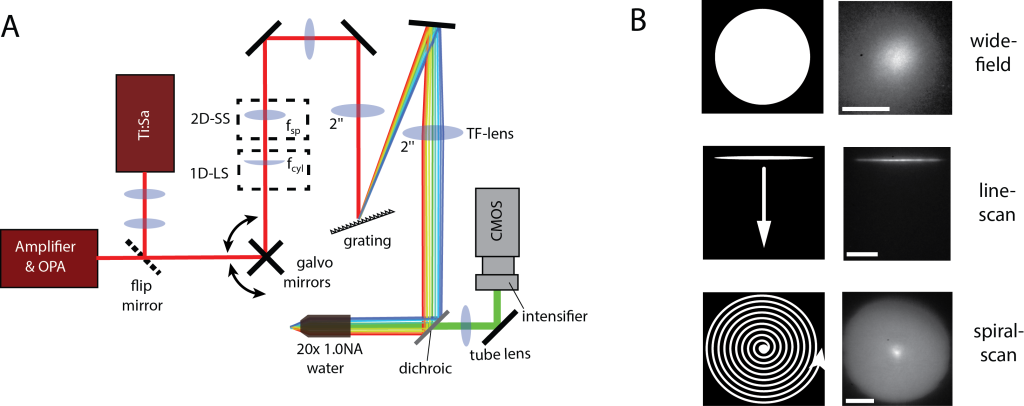
Our recent paper "Optimizing and extending light-sculpting microscopy for fast functional imaging in neuroscience" on the improvement of our previously published…January 9, 2015
The Vaziri lab has successfully secured with colleagues at IMP and IMBA two grants in the WWTF's Life Sciences Call…